20+ Calculating Heat Of Reaction From Constant-Pressure Calorimetry Data
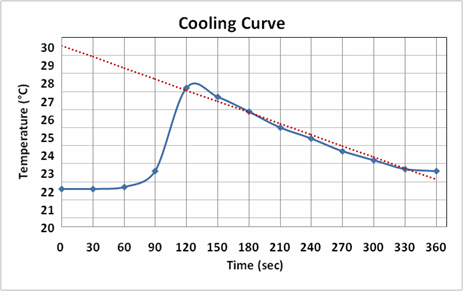
G of water at 200C. Maximum number of marks points should be joined such that a smooth curve called best fit curve or.

Schematic Of Constant Pressure Calorimeter For Apw Classification Download Scientific Diagram
Enter the email address you signed up with and well email you a reset link.

. Calorimeter 722 Hints and Explanation Principle of Calorimetry Specific Heat 750 723 723 Chapter 8 Wave Motion. ASTM D381 - Existent Gum Content. Calculating the limiting reactant the change in enthalpy of the reaction H rxn can be determined since the reaction was conducted under conditions of constant pressure H rxn q rxn moles of limiting reactant.
Data points for a calorimetry experiment plotted on a graph. Enter the email address you signed up with and well email you a reset link. Gas is one of the four fundamental states of matter the others being solid liquid and plasma.
Enter the email address you signed up with and well email you a reset link. In addition many applied branches of engineering use other traditional units such as the British thermal unit BTU and the calorieThe standard unit for the rate of heating is the watt W defined as one joule per second. 4Fe s 3O 2 g.
Analysis of H SS for 1176 binary metallic systems from Ref. 2C s H 2g C 2 H 2g. In one experiment 0558 g element X was found to react with 0320 g element Y to form only one product compound Z.
119 119 119 120. Enter the email address you signed up with and well email you a reset link. Carbon dioxideA gas mixture such as air contains a variety of pure gases.
We encourage teachers and other education stakeholders to email their feedback comments and recommendations to the Commission on Higher Education K to 12 Transition Program Management Unit-Senior High School Support. Are for mixing in the liquid state and the percentages of ideal and regular. Then we have to mark each data on the graph with a sharp marker.
14 mol C H 12011 g 10079 g 14007 g 18 mol H 2 mol N mol C mol H mol N 5 mol O b. Nelkon and Parkers A lvl physics Books for those who are going to be taking IGSCE and other equivalent Exams soonI do not hold any copyright or anythingJust wanted to share the free material for those who are keen to learn PhysicsAll Rights. 2 Q10 The equation for the enthalpy of formation of ethyne is.
The Bomb Calorimeter Model-IKA C2000 was used to measure the cross calorific value of the solid and liquid samples. Here the time is required and the total charge to complete the electrochemical reaction. Enter the email address you signed up with and well email you a reset link.
Oxygen or compound molecules made from a variety of atoms eg. 156 mol d. Key Terms and Concepts in Thermochemistry system surroundings internal energy and its relation to heat and work state functions Enthalpy exothermic and endothermic reactions and their application in calculating the heat of reaction Hesss law Heat Capacity and Specific Heat Specific heat capacity Calorimetry reactions under.
Here the time is required and the total charge to complete the electrochemical reaction. Pressure is recorded at regular intervals until a constant pressure is obtained. 50 mg e.
Use this information and the enthalpy of combustion of hydrogen in the data booklet to calculate the enthalpy of formation of silane in kJ mol-1. Use Hesss Law to calculate the heat of combustionof 1-octanol. Transcript 10 mol F2 440 g CO2 40 g H2 146 g SF6 34.
As a form of energy heat has the unit joule J in the International System of Units SI. 100 g C14H18N2O5 c. Conduct a laboratory session on the naming of compounds and on formula writing.
EVALUATION 20 minutes Check-up Quiz Choose the best answer from among the choices given. Here the reagent is a constant direct electrical current of known magnitude that consumes the sample. If the heat capacity of the bomb calorimeter is 420JC and the heat of combustion at constant volume of the sample is 3374kJmol calculate the final temperature of the reaction in Celsius.
How many grams of compound Z. Anna Brewer StudySmarter Originals. The first law requires that q surr q sys and at constant pressure q sys.
Free energy is a state function so its value depends only on the conditions of the initial and final states of the system. How much energy was. Here the reagent is a constant direct electrical current of known magnitude that consumes the sample.
This Teaching Guide was collaboratively developed and reviewed by educators from public and private schools colleges and universities. The data in Ref. Measurement Errors and Uncertainties Graphical Presentation.
Of Compounds data table. The specific heat capacity of water is 4184 Jg C. A pure gas may be made up of individual atoms eg.
Calculating Free Energy Change. ΔH -1517 kJ mol-1 The enthalpy of combustion of silicon is -911 kJ mol-1. This is called Plotting.
A 0500 g sample of C7H5N2O6 is burned in a calorimeter containing 600. This pressure is reported in psi and kPa. Calculate entropy changes for various processes eg isothermal process free expansion constant pressure process etc.
This involves measuring mass of reagent that reacts completely with the sample. It is a constant-volume type calorimeter that measures the heat of a particular reaction or measures the calorific value of the fuels. Ideal and regular solutions are uncommon.
The sample is cooled placed in the test chamber and brought to the test temperature. This involves measuring mass of reagent that reacts completely with the sample. The symbol Q for heat was introduced by Rudolf.
Atom pairs with H B C N O P and S are excluded in the present analysis shows that only 4 and 11 of the systems are ideal and regular solutions respectively. A noble gas like neon elemental molecules made from one type of atom eg. HClaq NaOHaq -- NaClaq H 2 Ol Energy.
Thermochemistry determine the heat exchanged at constant pressure q m c T. The specific heat capacity of the water is 418Jg-1 K 1. Anand in Advances in Eco-Fuels for a Sustainable Environment 2019 13254 Calorific value.
Popular chemical hand warmers generate heat by the air-oxidation of iron. Zeroth law of thermodynamics and Temperature measurement 2. Fuel X was burned in a calorimeter and 50g of water rose from 202C to 45C.
Heat and heat capacity 4. Enthalpy change is the heat change of a reaction at constant pressure. 15999 g 294305 gmol mol O 1 mol C14 H18 N 2 O 5 340 102 mol C14H18N2O5 2943 g C14 H18 N 2 O 5 2943 g 459 g C14H18N2O5 mol 1g 1 mol 602 10.

Constant Pressure Calorimetry Introduction To Chemistry Course Hero

12 3 Heat Capacity Enthalpy And Calorimetry Chemistry Libretexts

Chemistry 1202

Constant Pressure Calorimetry
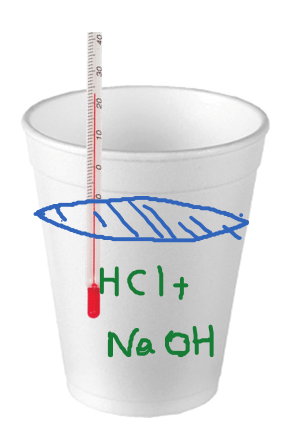
Constant Pressure Calorimetry Chemistry Steps

Chemistry 101 Constant Pressure Calorimetry Youtube

Survey On Stochastic Distribution Systems A Full Probability Density Function Control Theory With Potential Applications Wang 2021 Optimal Control Applications And Methods Wiley Online Library
Solved Calculating Heat Of Reaction From Constant Pressure Calorimetry Student Dissolves 12 6 G Of Ammonium Chloride Nhaci In 250 G Af Water In Well Insulated Open Cup She Then Observes The Temperature Of The
Which Energies Are To Be Added Or Subtracted In A Born Haber Cycle Quora

Chemistry 101 Calculating Heat Capacity Of A Bomb Calorimeter Youtube

Solved A Calorimeter Is An Insulated Device In Which A Chegg Com

Coffee Cup Calorimeter Calculate Enthalpy Change Constant Pressure Calorimetry Youtube

Pdf Microbial Electrochemical And Fuel Cells Heillen Calderon Castillo Academia Edu
Lab Background
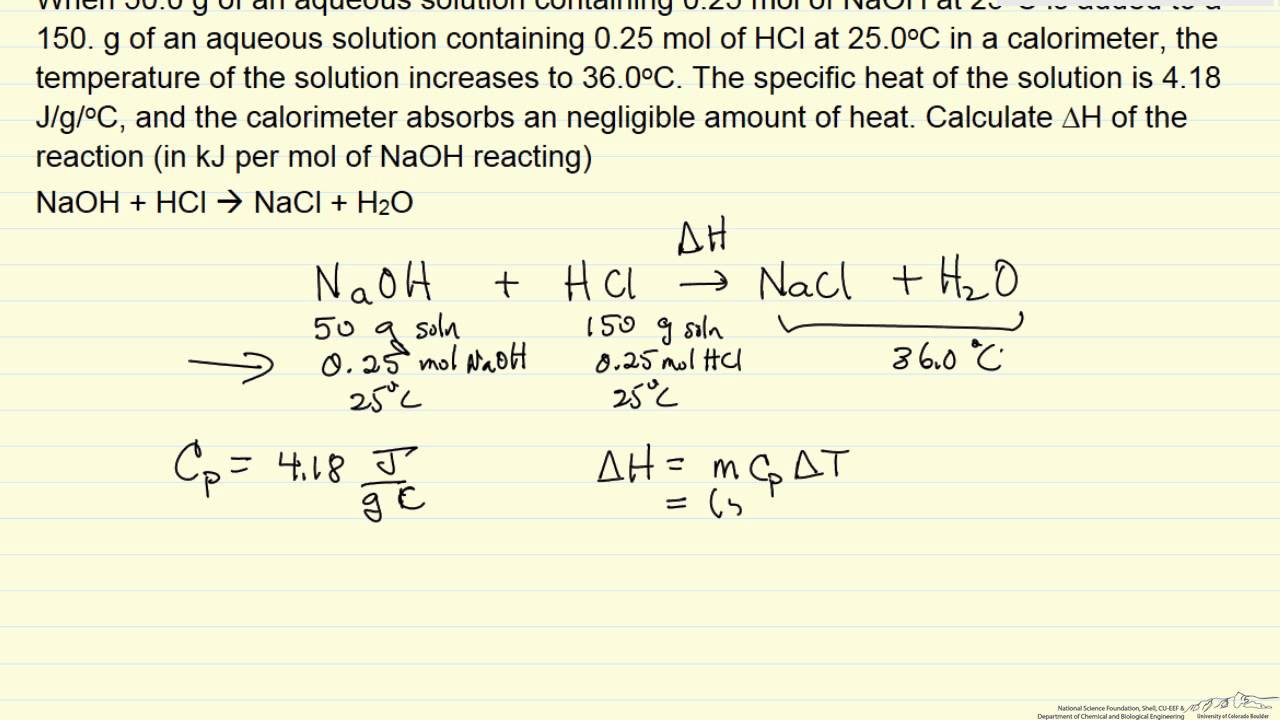
Heat Of Reaction From A Calorimeter Example Youtube

Chem10003 Physical Chemistry Notes Chem10003 Chemistry 1 Unimelb Thinkswap
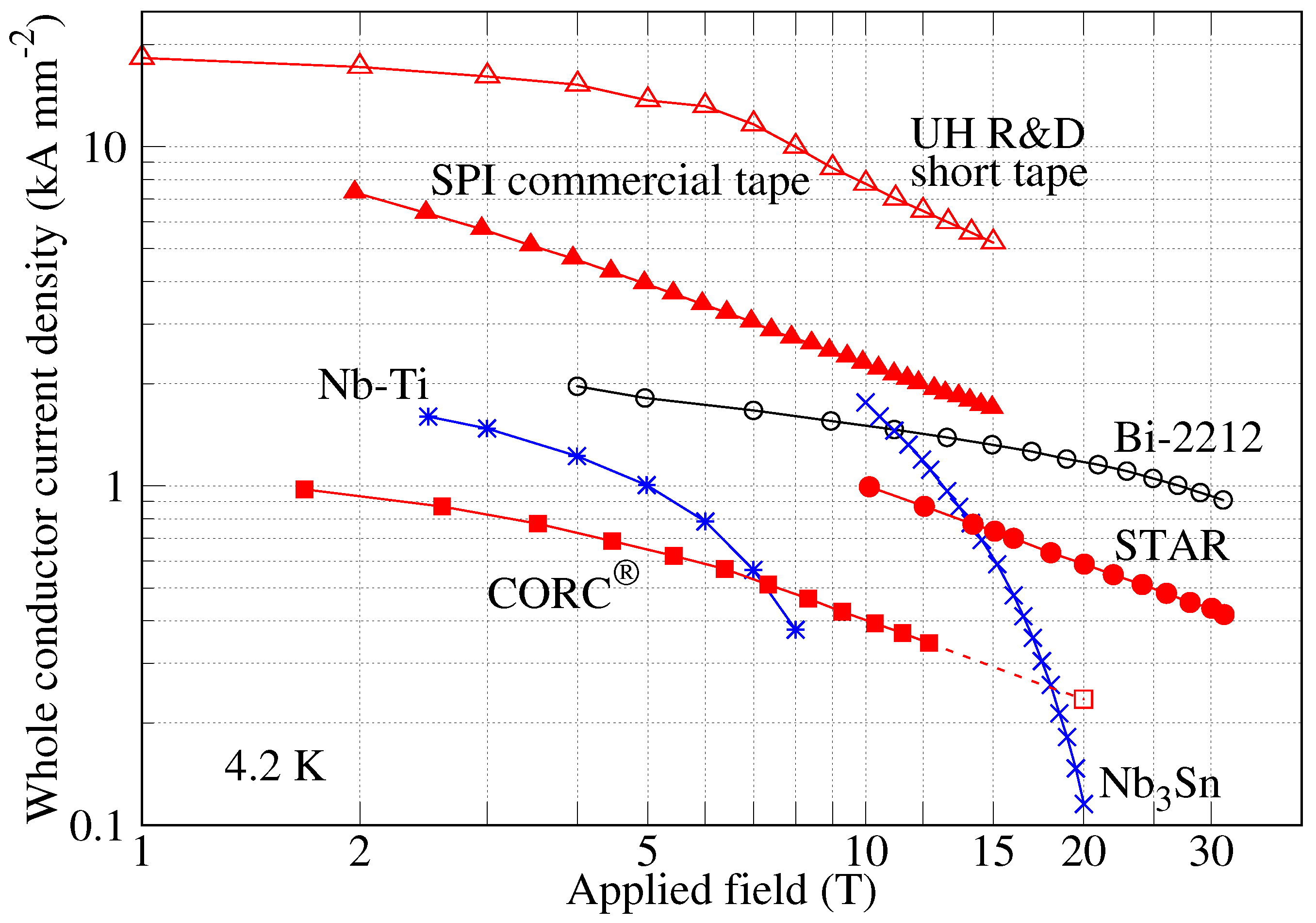
Instruments Free Full Text Dipole Magnets Above 20 Tesla Research Needs For A Path Via High Temperature Superconducting Rebco Conductors